IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 35 Issue 3 (October 2018)
Citation: De Luca, DW, Dewas, M, Mpunga, N, Machaga, SJ and Phillipps, GP (2018). The Conservation Status of Otters in Southwest Tanzania. IUCN Otter Spec. Group Bull. 35 (3): 134 - 147
The Conservation Status of Otters in Southwest Tanzania
Daniela W. De Luca1*, Maeva Dewas2, Noah Mpunga3, Sophy J. Machaga3, Guy Picton Phillipps4
1 Wildlife Conservation Society (WCS) P.O Box 922 Zanzibar, Tanzania e-mail: ddeluca@wcs.org
2 742 Chemin du Perdigal, 34820 Assas, France
3 P.O.Box 1475 Mbeya, Tanzania
4194 Westward Road, Ebley, Stroud, GL5 4ST UK
Received 23rd November 2017, accepted 12th December 2017
Published previously as an internal report: De Luca, D.W., Dewas, M., Mpunga, N.M., Machaga, S.J., Picton Phillipps, G. (2017). The Conservation Status of Otters in Southwest Tanzania. WCS Report. pp 15.
Abstract: Two species of otters are confirmed in Tanzania, the African clawless (Aonyx capensis) and the spot-necked (Hydrictis maculicollis). Both are listed as ‘near-threatened’ in the IUCN Red List, with the main threats linked to pressures exerted on their habitat and food resources by a growing human population. Despite scant details of their Tanzanian distributions, our recent work shows that both species are more widespread than previously believed. We investigated distribution and conservation status in two highly populated areas in southwest Tanzania, Mt. Rungwe and Sao Hill. Rainy and dry season surveys for spraint sites included rivers, lakes and swamps, with data collected on associated dietary items and habitat features. Results indicated that species presence and distribution are not significantly affected by seasonality and that they can thrive as long as retaliatory hunting is prevented. Habitat quality should be monitored to avoid deterioration of escape cover. The nature of threats and the conservation of both species are discussed.
Keywords: clawless otter, spot-necked otter, Tanzania, status, threats
INTRODUCTION
Otters are top predators in a variety of aquatic and semi-aquatic habitats (Foster-Turley et al, 1990; Kruuk, 2006). In sub-Saharan Africa among the three species present (Aonix capensis, Aonyx congica and Hydrictis maculicollis), two A. capensis and H. maculicollis are confirmed in Tanzania (Nel and Somers, 2002; Somers and Nel, 2013; Foley et al., 2014.). Aonyx prefer walking in shallow waters where they feed on crabs and other crustaceans, insects, frogs, and sometimes fish. Hydrictis meanwhile, has webbed feet for swimming in lakes and marshes or large rivers while feeding predominantly on fish. The absence of competition between the two sympatric species is due to their dietary specialisations (Somers and Nel, 2013; d’Inzillo Carranza and Rowe Rowe, 2013).
African clawless and spot-necked otters are both listed as ‘near-threatened’ in the IUCN Red List (Jacques et al., 2015; Reed-Smith et al., 2015). However, A. capensis is becoming extirpated in many localities and H. maculicollis is declining across its range. With the exception of South Africa (Nel and Somers, 2007; d’Inzillo Carranza and Rowe-Rowe, 2013; Somers and Nel, 2013) details on distribution, status and ecology are poorly known (Nel and Somers, 2002). In Tanzania, there is limited information about otter ecology (Kruuk and Goudswaard, 1990; Amulike et al., 2012), although some information on species presence and distribution is available from inventories (De Luca and Mpunga, 2005; 2006; 2013; Pettorelli et al., 2009; Foley et al., 2014). Despite sketchy details, our most recent distribution maps reveal that A. capensis is more widely spread than assumed (Foley et al., 2014). The presence of H. maculicollis is linked to permanent water with fish, and in Tanzania it is found in all major lakes, but only in the less disturbed areas (Foley et al., 2014).
The main threats are linked to an increasing human population (Rowe-Rowe, 1986; Nel and Somers, 2002) resulting in habitat fragmentation and degradation through removal of riparian vegetation. Morever, overgrazing and unsound agricultural practices (Mason and Macdonald, 1986; 1990; Rowe-Rowe, 1986; Kruuk, 2006; Somers and Nel, 2013) can pollute rivers and affect the prey base (Kubheka et al., 2012). African otters are not persecuted substantially for their fur, except in Ethiopia (Nel and Somers, 2002), but retaliatory and subsistence hunting occurs, and the use of body parts for medicines continues (De Luca and Mpunga, 2004; 2006). The challenge for conservation is to understand if/how African otters can adapt to increasing anthropomorphic pressures and how much habitat destruction and degradation they can tolerate. This study addressed these issues by assessing the status, distribution and ecology of both species in two areas of southwestern Tanzania, with emphasis on the threats facing the species.
METHODS
Study areas
The first area was in and around Mt. Rungwe Nature Reserve including parts of the Kitulo Plateau National Park. This comprised 150 km2 between 9°03’- 9°12’South and 33°35’ - 33°45’ East with elevation from 1500-2981m. Rainfall ranges between 1550 and 1850 mm/y, with the dry season between June and October. The habitat along Mt. Rungwe’s rivers is of two main types: a mosaic of dense, high secondary riparian vegetation deriving from re-colonisation of abandoned cultivation or fire climax grassland, and cultivation extending to the bank and mixed with planted trees. Bushed, woodland, and in less disturbed areas montane forest, may occur along the rivers. Above 1900m, montane grassland reaches the river banks. At lower altitudes, large strips of reeds and tall grasses are found. No resident large carnivore species were present except for transient leopards that do prey on otters (De Luca and Mpunga, 2018).
The second area was Sao Hill, in Mufindi District, Iringa Region, approximately 2500km2 between 8°25’- 8°95’ South and 34°76’ - 35°74’ East. This is a highland area including plateau, and has one of the coolest and wettest climates in Tanzania. The landscape is dominated by tea plantations including intact and well-preserved clusters of natural forest (relict patches of ‘Eastern Arc Mountain’ forest), and some commercial pine, eucalyptus and cypress plantations. Large natural and man-made lakes, swamps, and about one hundred small man-made (tea estate) dams linked to a network of small rivers offer a rare opportunity to study these co-existing otter species. No large predators are resident.
Surveys
Mt. Rungwe
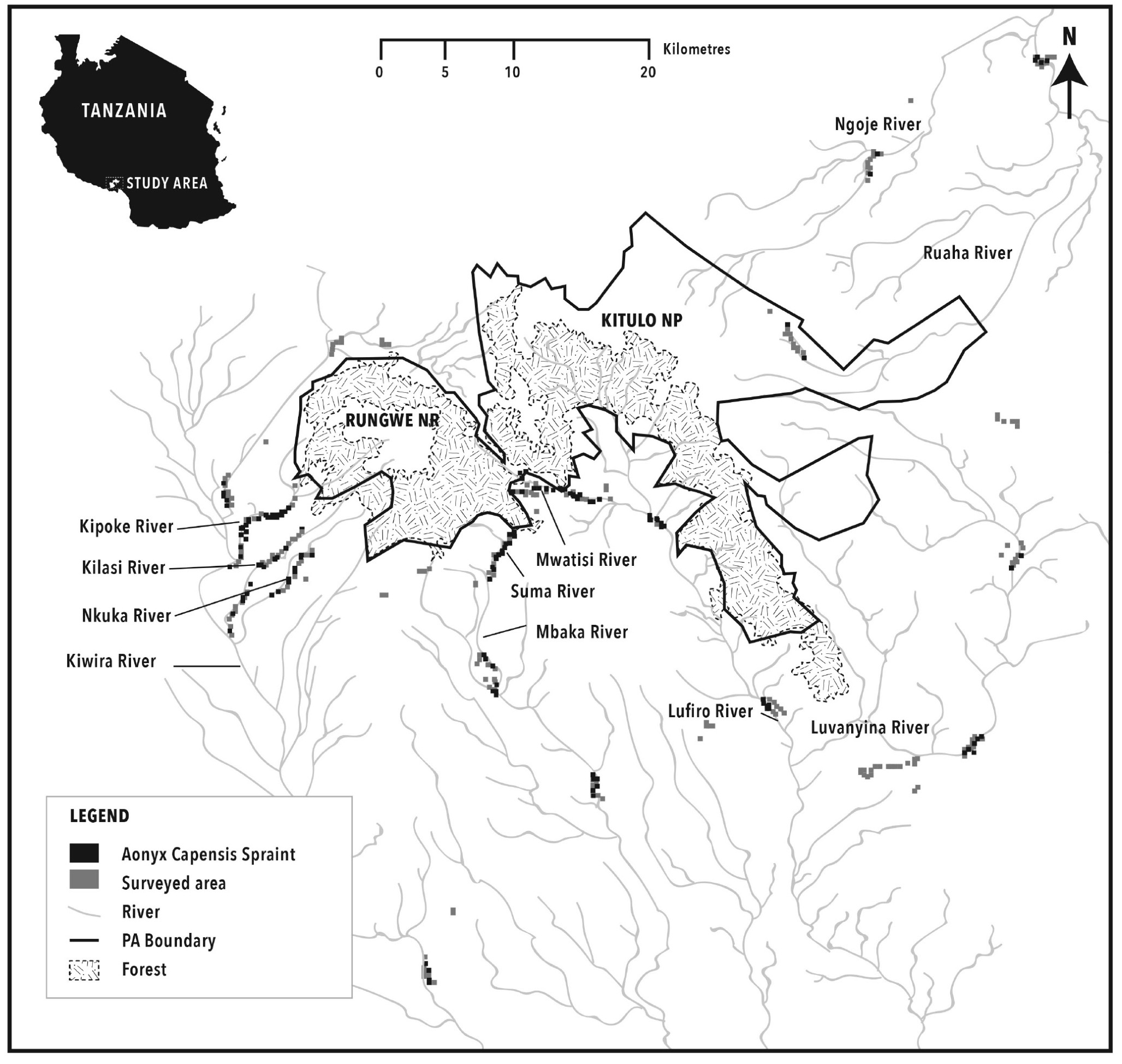
Using a map of the Mt. Rungwe/Livingstone/Kyela Basin, developed from ASTER Global Digital Elevation Model (30m) (Fig. 1), four water catchments were chosen to investigate otter presence and distribution. These were Kiwira, Lufirio, Mbaka, Ruaha; the first three entering Lake Nyasa/Malawi. In each catchment, different portions of rivers were intensively searched for otter signs (holts, rolling places, runs, spraint sites and tracks) between January and March 2010 (wet season) and August and September 2010 (dry season) (Table 1). Only two of the river sections surveyed were within protected areas: the Kalambo river, and the upstream part of Ndala river in Kitulo NP. Half of continuous river sections surveyed were between 2-3km, 28% were 1-2km and around 20% were 3-3.8km in length, following Rowe-Rowe’s (1992) recommendations. In each catchment, sections at low, medium and high altitude were surveyed ranging from 600m to 1200m and 1800m asl except for Kilasi at Ruaha which reached 2500m (mean 1465 m; min 580 and max 2570 SD 501).
Both river banks were surveyed by teams of two/three trained observers and all otter signs were recorded. These consisted of spraints diagnostically identified by size, diameter, shape and smell (Rowe-Rowe 1977a). Spraint sites were categorized as ‘latrine’ or ‘non-latrine’ according to the number of scats found together (1 or more clustered within 50 cm2). Spraint age was recorded as very fresh (1 day old), fresh (2-3 days old) or old (more than 3 days). The diameter of intact spraints (mostly the very fresh and fresh ones) was measured with calipers.
In order to identify factors associated with habitat preference, we recorded: site location (river bank, island, other), river configuration (riffles, water surrounding island, deep flowing water, junction, pool, shallow flowing water and rapids), substrate of deposit area (vegetation, mixed, bare soil, rock, litter), habitat (vegetation types; grassland, woodland/grassland mosaic, swamp, weeds, marsh, rocks) and cover type, human disturbance (roads or paths close to the river, human activities in and along the river). River characteristics (width, depth, bottom, speed), bank height and slope, and distance to water were also noted.
Sao Hill
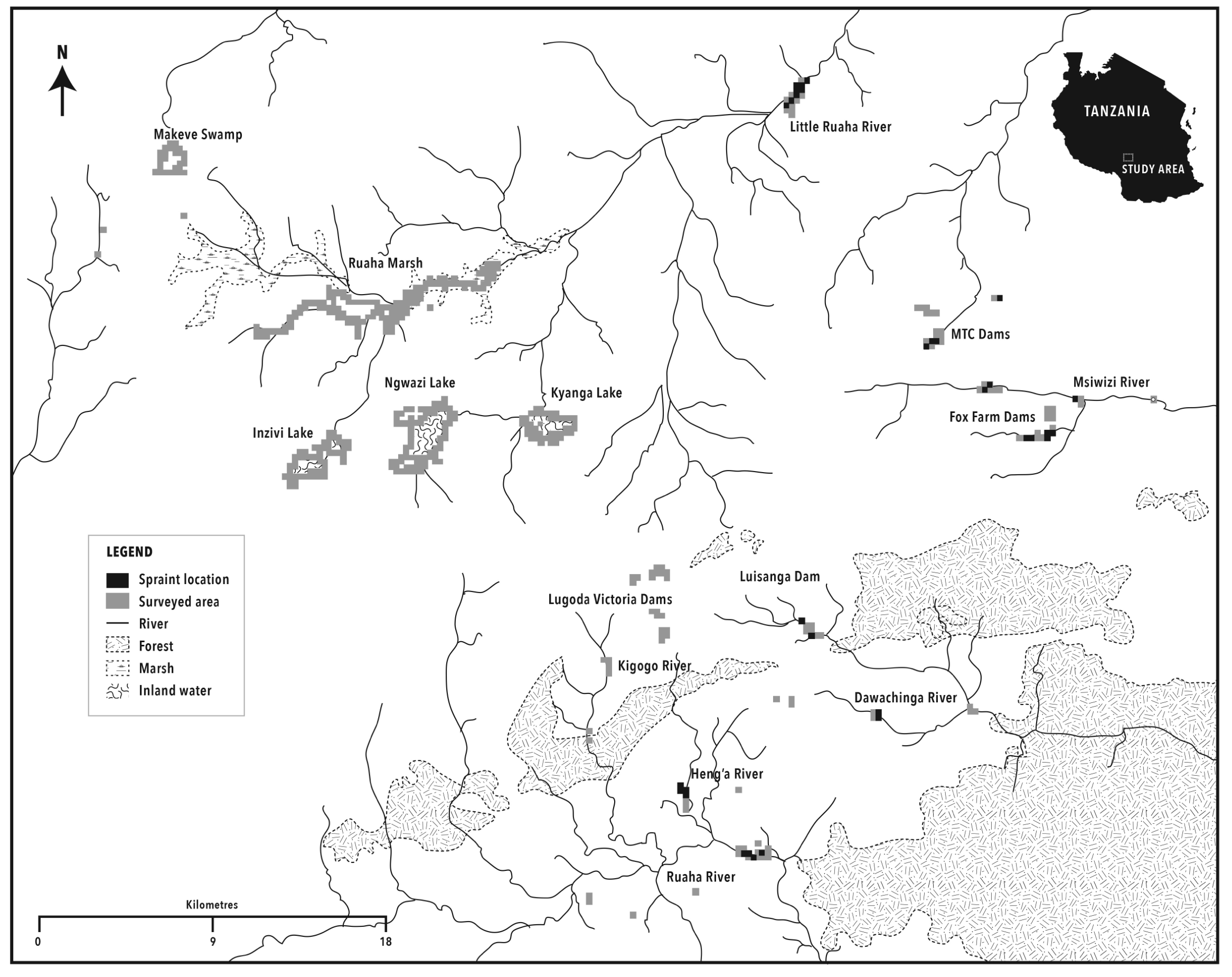
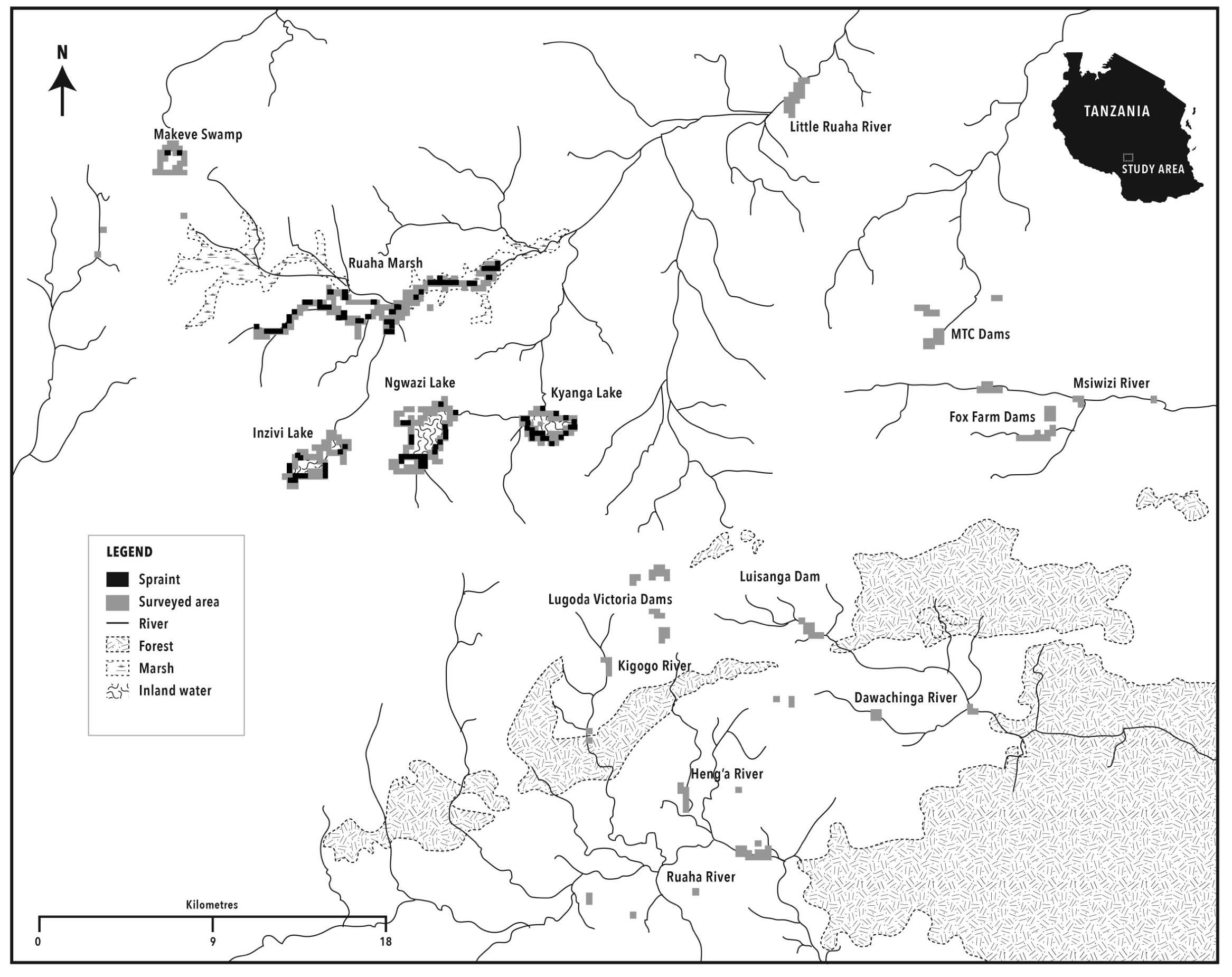
A map of Sao Hill, developed from ASTGTM Global Digital Elevation Model (30m), was used for survey planning (Fig. 2). Work was divided into three sessions. Large lakes and swamps (n=5) in Sao Hill, Lake Ngwazi, Ruaha Marsh, Lake Inzivi, and Lake Kyanga spending an average of 3 days in each site. Surveys were carried out on foot along the shore but canoes were also used to access island shores and ‘floating islands’ of grass. Large parts of the lakeshores consisted of dense riparian grasses, natural thick bush and shrub land. Other parts were short grasses, accessible to humans and cattle. Marshes created a transition zone between water and land.
Secondly, a small swamp connected to the large Ruaha Marsh, and a sample of small man-made dams in the tea estates and private tourist lodge (Fox’s Farm) were surveyed. Finally, 12 other small tea estate dams were surveyed by canoe. A sample of river sections (n=7 of 1 km in length), part of a wide and complex network of rivers (permanent and seasonal), belonging to two water catchments and connected to the small dams and the large Ruaha marsh, were surveyed for otter sign on both riverbanks on foot. In each catchment, 1 to 3 river portions (1 per river) were visited (Fig. 2). The altitude ranged from 1200-1300m asl in the river sections and 1700-1900m asl in the swamps and lakes, man-made dams, and rivers on the high plateau of Sao Hill. The average water depth of large lakes and swamp, excluding Mkewe Swamp, was 2.15 ± 1 (range: 0.6-3.8m; bimodal: 1m and 3m; 80% between 1 and 3m). Differences in site density between dry and wet seasons were tested for significance using the t-test.
Initially to verify activity patterns of both species, we deployed 6 Leaf River (Leaf river outdoor products) camera traps along the lake Ngwazi shore for 5328 hours, they were set at 300m interval distance from each other.
Interviews
Structured interviews were carried out to compile complementary data on otter presence and behaviour, and threats. The first part of the interview assessed villagers’ perceptions and exploitation. The second part investigated river exploitation by fishermen and the presence of fish and crab species. The third part of the interview dealt with agricultural up to the river edge. This type of interview was adapted to Sao Hill fisherman but originally developed for Mt. Rungwe.
RESULTS
Distribution and site density of A. capensis spraints in Mt. Rungwe
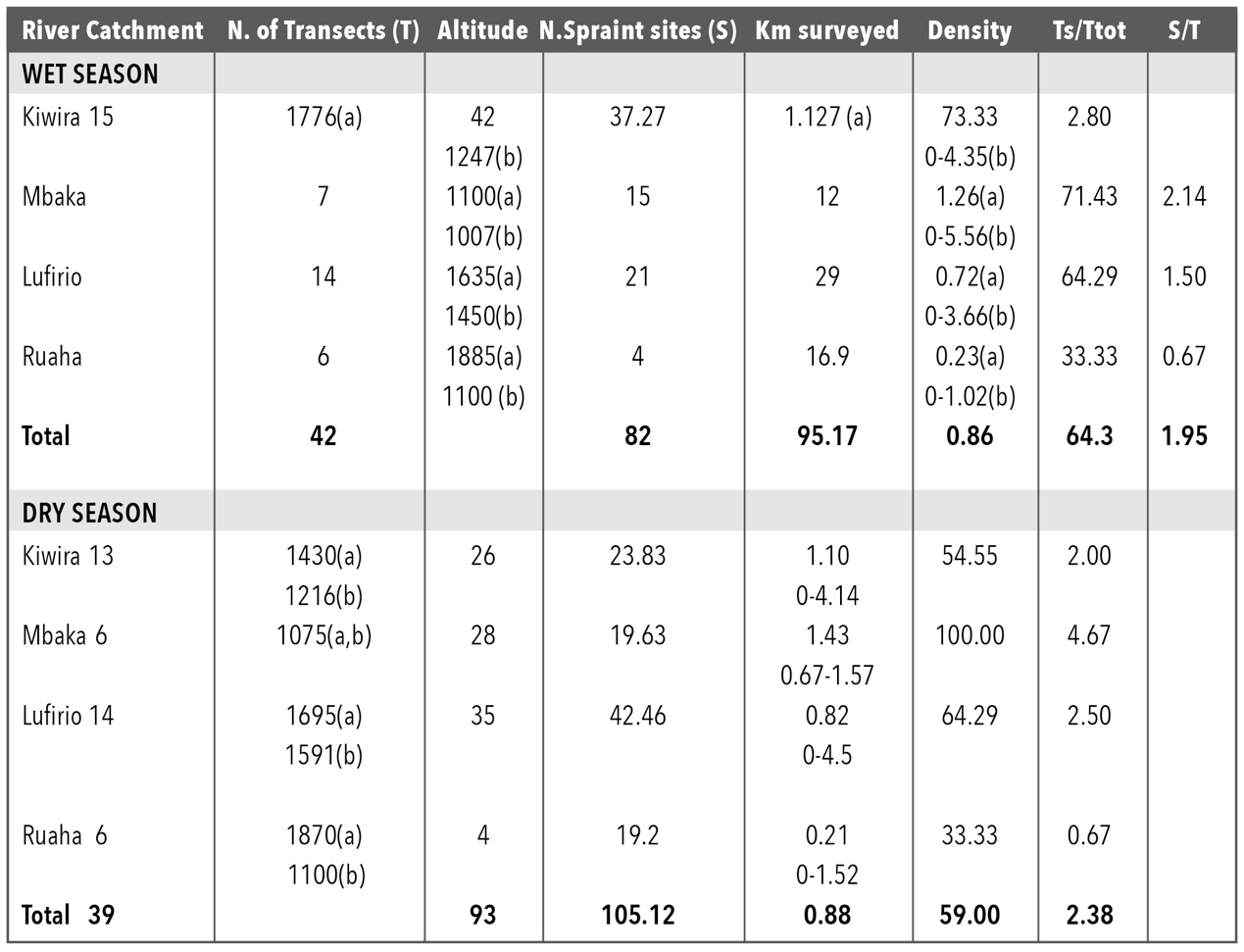
Clawless otter sites were found on 64.3% of the 42 river sections in the wet season and on 59% of 39 river sections in the dry season; overall 62% of the transects surveyed were positive for the presence of A. capensis (Table 1). A. capensis distribution along the rivers showed the richest sites in terms of density (Table 1, Fig. 1). Otter site density above 1 per 1 km was recorded between 700 and 1650m asl, with the highest densities recorded at 1000 and 1500m (Table 1). Site density values did not differ significantly between the two seasons (t-test two sample for mean =0.42, df 41, P=NS). For spraint site abundance or frequency of sprainting, the difference was not statistically significant between seasons (t-test, two samples for mean =-1.13, df 4, P=NS). The mean altitude of the transects surveyed was 1600m asl in the wet and 1520m asl in the dry season, however, otter sites were concentrated around 1200-1245m asl.
Features and habitat description of A. capensis spraint sites
During the wet season, of the 81 spraint sites located on 42 river sections within 10 different rivers, 80% were in latrines. Of these, 45% were regularly used, and about a third were found along Kipoke River (Fig. 1). Only 17% were isolated scats, mainly old. During the dry season, the number of isolated scats doubled to 34.85%. Out of 3 holts found, during the study only one was recorded as in use; located on the bank of Kipoke River under large rocks in dense vegetation. Two other holts were found, one along Suma and the other along Lufirio.
The habitat features of the sites did not differ significantly between wet and dry season (all t tests paired: type of scats: latrine /single scat t=-0.63, df1, P=NS; site location: river bank, island, others, t=-0.33, df 2, P=NS; configuration: riffles, water surrounding, island, deep flowing water, several, junction, pool, shallow flowing water, rapids t=-0.43, df 7, P=NS; substrate: vegetation, mixed, bare soil, rock, litter, t=-0.54, df4, P=NS; vegetation type: rocky bushed grassland, bushed grassland, grassland, bamboo, eucalyptus, agriculture, riverine forest, weed grasses, reeds vegetation, tangled bush vegetation, tangled bush grassland, pine woodland; secondary riverine forest with or without tangled vegetation t=-0.3, df12, P=NS; distance to water: <5,6-10 m, 17-90 m, 60-90m, t=-1.38, df3, P=NS).
Spraint sites were mainly located on river banks and associated with three river configurations: beside rivers, a riffle configuration (rockier bottom), water surrounding islands and deep flowing water. Otters mainly deposited spraints in ‘bushed grassland’, ‘grassland’, and ‘rocky bushed grassland’ but some were found amongst eucalyptus and cultivation.
Aonyx capensis distribution and density in Sao Hill
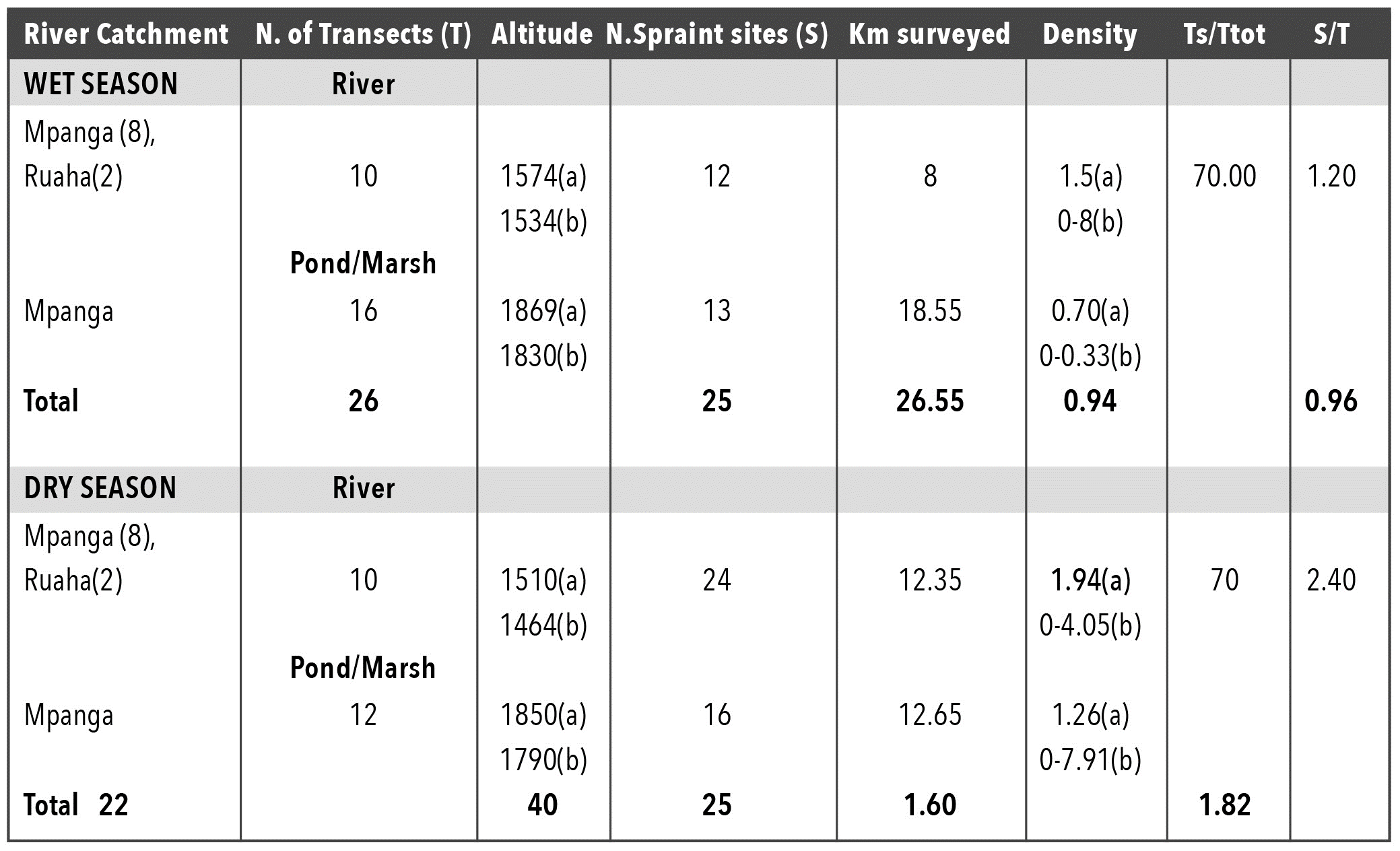
In the wet season 46% of transects around ponds, dams and along rivers were positive for A. capensis (N=26 transects), while in the dry season 50% showed signs of A. capensis (N=22 transects). Signs were found on only four out of 12 dams surveyed (Table 2). In both seasons around 70% of the scats were found in latrines hence there was no significant difference between seasons (t-test =3, df1, NS). No statistically significant difference was recorded in density and abundance of A. capensis between seasons (density: t-test =0.41, df 41, P=NS) (abundance: t-test: two samples for mean =2.53, df 1, P=NS). Figure 2 shows the spraint sites on rivers and dams. The mean altitude of transects and that of sites with otter scats in the wet and dry seasons did not differ more that 60m in altitude, however the altitude of the sites ranged from 1200-1950m asl in both seasons.
Habitat description of A. capensis sites in Sao Hill
For none of the features was there a significant difference between wet and dry seasons: (all t-tests paired: type of scats: latrine/single scat t=3, df1, P=NS; site location: river bank, swampy edge, island, path t=-1.28, df 6, P=NS; configuration: swampy river, shallow flowing water, pool, junction, riffles, unknown, t=1.17, df 6, P=NS; substrate: vegetation, rock, bare soil, mix, litter t=-1.57, df4, NS; vegetation type: woodland grassland mosaic, montane forest grassland mosaic, swamp, weeds, marsh, rocks and grassland, rocks, t=-0.77, df7, P=NS; distance to water: <4 m, 4-10 m, 15+m, t=-1.8, df ,2 P=NS; distance to cover: <1 m;1-2.5m, 30-80m, t=-1.9, df2, P=NS). In both seasons, sites were found mainly in latrines, on the river banks, on vegetation as a substrate, at less than 4m from water and at less than 1m from cover. In the wet season, sites were found in a variety of habitats whereas in the dry season, they were mainly in woodland grassland mosaic and grassland. The river configurations were deep-flowing water and swampy river. Human disturbance (fishing, tourism, canoeing and timber gathering) was present at all times.
H. maculicollis spraint site distribution and density in Sao Hill
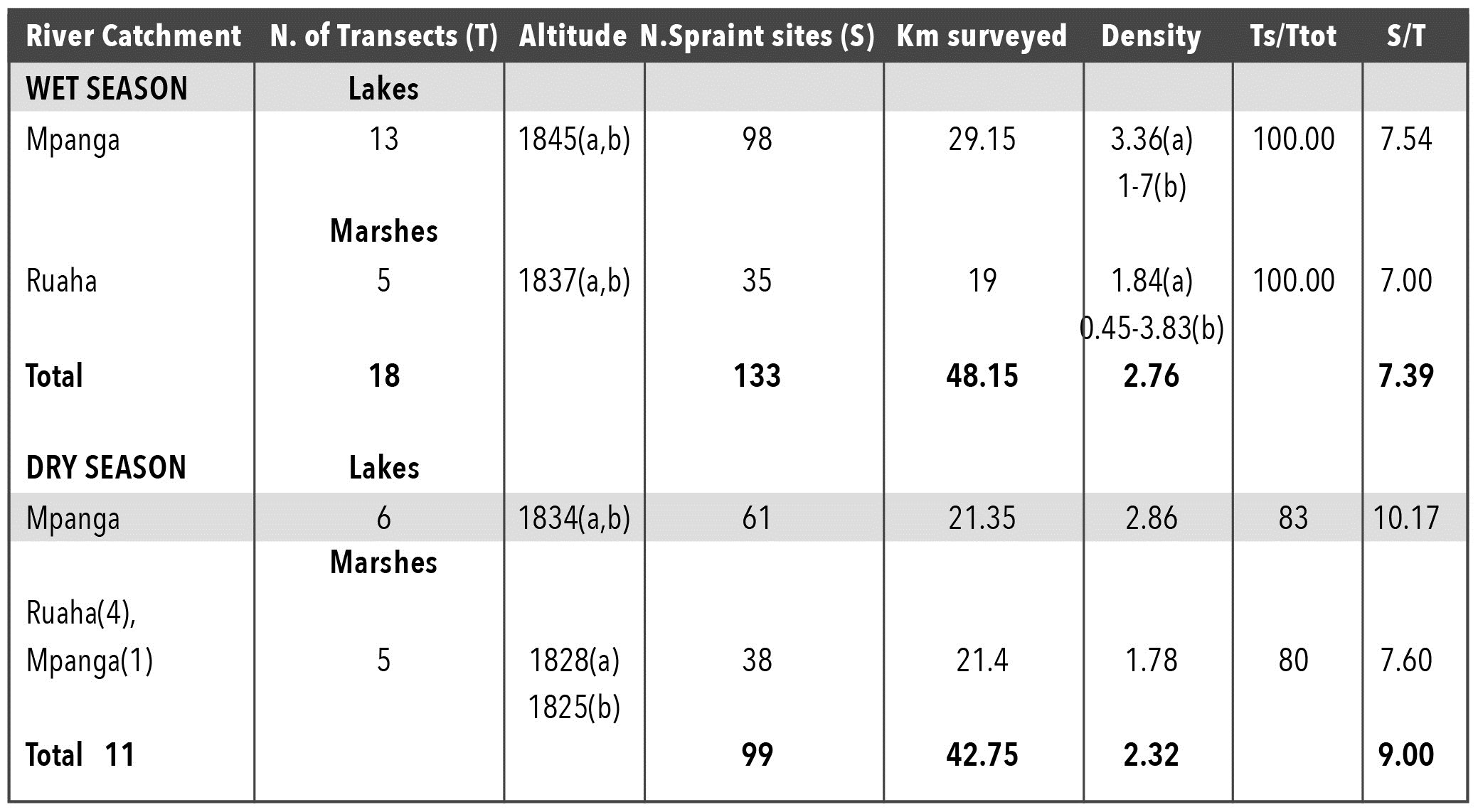
Figure 3 shows the distribution of spraint sites in Sao Hill along lakes, swamps and marshes. In the wet season all sites visited were positive for H. maculicollis, while in the dry season 80% in the lakes and 83% in the marshes were positive. Towards the end of the wet season, 133 H. maculicollis spraint sites were distributed between the three lakes and the two swamps surveyed (Table 3). Also for H. maculicollis site density and abundance or frequency of sprainting, there was no significant difference between seasons (density: t-test =0.92, df 24, P=NS), (abundance: t-test two samples for mean =1.6, df1, P=NS). The areas (lakes, Ruaha Marsh, and the swamp) where H. maculicollis presence was recorded ranged from 1826-1857m asl, with a mean altitude of 1845m asl (Table 3).
Habitat description of H.maculicollis spraint sites in Sao Hill
For none of the habitat features there was a significant difference between seasons (all t test paired: sign location: island, swampy edge, lake shore, marsh t=1.77, df 3, P=NS; substrate type: vegetation, rock, litter t=0.99, df3, P=NS; vegetation type: marsh, grassland, woodland grassland mosaic, rocks t=0.21 df 4, P=NS; water depth: t= 0.68, df 3, P=NS).
H. maculicollis spraint sites were found in latrines in 76% of the cases during the wet season (N=130), and for 62% in the dry season (N=99). Spraints were deposited in both seasons on the islands and small floating islands within the lakes, on vegetation in marshes and swamps. In the swamp, sites were found on waterlogged grassland between 12 and 40m from the main water body. Signs of otter activity were often observed and most were in the vicinity of human disturbance (fishing or cattle grazing) or paths, but none were found on the vicinity of cultivated land against the water’s edge.
Spraint diameters
The mean diameter of A. capensis spraints in Mt. Rungwe measured 24.7 ±0.45mm (n=25; range 15-35mm). In Sao Hill the diameter (N=23) ranged between 20 and 28mm. In the small dams, A. capensis mean spraint diameter measured was 22.4mm ±2.1 (n=5; range 20-25 mm) and, for the rivers, mean spraint diameter measured was 22.8mm ±2.1 (n=18; range 20-27 cm). Confirming that H. maculicollis is occupying the lakes and swamps in Sao Hill, the mean spraint diameter was 16 ±4.2mm (n=10; range 10-23mm) with only one scat having a diameter >20cm.
Preliminary food contents observations in Sao Hill
In the small dams within tea estates, A. capensis spraints (N=5) containing fish only, were found as well as spraints containing only crabs (Potamonautes sp.) (N=4). Five contained both fish and crabs. Fewer species of fish inhabit the small dams, and they belong to the family and genera below, except for one dam (on the Fox Farm), which had introduced rainbow trout (Oncorhynchus mykiss). A. capensis was observed (Fox Farm) eating trout.
The majority of H.maculicollis spraints in large lakes and swamps contained fish (scales, bones) and jelly. On the islands fish remains were found. During the wet season in Lake Inzivi 23 spraints, and in Lake Kyanga 9 spraints contained fish and crabs. By collecting fish specimens and by interviews, we identified 5 fish families: Kneriidae (Kneria sp.), Cyprinidae (Barbus sp.), Cichlidae (Tilapia sp.), Clariidae (Clarias sp.), Centrarchidae (Blackbus sp.) in the four major lakes and swamps. Local fisherman identified the fish remains as catfish, Clarias sp. (n=7), and Tilapia sp. (n=4). The same fish genera were present in surveyed rivers (except for Kneria sp. and Blackbus sp.).
DISCUSSION
This study provides detailed information on the distribution, abundance and ecology of A. capensis and H. maculicollis in southwest Tanzania. The use of spraints to monitor distribution and abundance, despite being questioned (Kruuk and Conroy, 1987), is effective, repeatable and sustainable, providing there are trained technicians (Mason and Macdonald, 1987). With some precautions, the density of signs can be used for broad comparisons of populations. Otters use spraints for signaling to other individuals about resource availability, and thus more are deposited when resources are already being used in Lutra lutra (Kruuk, 1992). Both sexes deposit spraints at the same rate on territory boundaries (Kruuk, 1992) and a decrease in sprainting activity has been observed by Carss and Parkinson (1996) during the mating season. Seasonal cycles in sprainting activity linked to marking behaviour and food availability can affect the number of signs found. Results from this study showed no significant difference between seasons on the number of A. capensis and H. maculicollis spraint sites in both study areas. Our minimum transect length was above 200m and often exceeded the 600-1000m used in European surveys (Mason and Macdonald, 1987). Being the first study of these species in these areas we searched a minimum of 1km (mean transect length 2.5km) on both banks of the rivers in Mt. Rungwe and Sao Hill to confirm presence of A. capensis, and >3 km (3.3km mean) for H. maculicollis.
Mt. Rungwe catchment areas
Mt. Rungwe is the wettest area in Tanzania and an evergreen forest (Davenport et al., 2010). Heavy rains and floods may affect the probability of finding sites in the wet season (Rowe-Rowe, 1992), indicating that our data from the wet season may have been underestimated. In Mt. Rungwe, many A. capensis sites were recorded in every stretch of river surveyed. Over the two sessions an average of 0.875/km otter sites was found in non-protected areas, in a highly human dense part of Tanzania, unlike studies in South Africa (Perrin and Carugati, 2000a).
We recorded an altitudinal interval of species distribution in the area: spraint sites ranged from 1041-1520m asl, having sampled from 580-2570 m asl. A similar association between presence and altitude mid-river was recorded in South Africa with presence linked to higher central productivity (Nel and Somers, 2007). The number of spraint sites recorded as latrines did not change between seasons but those sites recorded as single, doubled in the dry season. It is possible that being the time of dispersal, single individuals were roaming further from holts. Dry-season sampling was conducted in mid-August and September, which is the time that after ‘winter’, temperatures are increasing. This period is known to be when mating and dispersal occurs in South Africa (Perrin and Carugati, 2000a;b; Rowe-Rowe, 1992).
Perrin and Carugati (2000a) emphasized the role of dense cover provided by tall grasses and sedges rather than bushes over riverbanks for both species. Similar results were found in habitats in southern Africa (Kamberg Nature Reserve, Natal, Rowe-Rowe 1992; Eastern Zimbabwe: Butler and Du Toit 1994). In this study, riparian vegetation was heterogeneous with large parts of riverbanks covered by dens, tangled vegetation and tall grasses offering cover. Other features recorded in other studies (Perrin and Carugati, 2000a) are river types such as the riffle (rockier bottom), in which A. capensis can easily see and chase prey, and water surrounding islands which provide cover to rest, hide and a location for holts.
Threats in Mt. Rungwe
The threats to A. capensis in Mt. Rungwe are of two types, persecution by hunters, and habitat destruction (including water quality) linked to agricultural practices. Information concerning hunting was provided voluntarily by 3 people out of 22, who admitted hunting otters opportunistically. Around 50% of those interviewed mentioned that hunting was common more than five years before. In a carnivore study in Mt. Rungwe in (De Luca and Mpunga, 2013), 56 (44.4%) of interviews answered questions about hunting. They claimed hunting for carnivores occurred mainly in the 1970s and 1980s. A. capensis was hunted with log traps, its skin valued for medicinal and witchcraft uses, the former included treating neck and back pain, epilepsy, convulsions and mental illness. Otter blood is believed to increase fighting strength of children. In South Africa otter blood is used to increase fighting strength, but of dogs (D’Inzillo Carranza pers. comm.). Until the 1980s in Mt. Rungwe members of some royal clans used to be buried in otter skins (De Luca and Mpunga, 2012).
Habitat destruction is caused by cattle grazing, wood cutting, charcoal production and fires set to clear old cultivation. These activities increase the risk of erosion and water siltation, and interfere with otter holts, water clarity and prey visibility. Rivers in Mt. Rungwe are often illegally cultivated up to the banks. Some cultivations are not cleared yearly favouring the regrowth of tangled vegetation, which provides cover to otters. Once the crop is harvested in the dry season and the fields are left to rest, our data (84.7% of spraints) suggest that otters did not deposit spraints near cultivated land. Otters are able to live between cultivated lands in riverbank pockets, which provide enough cover. Here, A. capensis does not appear to be affected by human activities such domestic laundry and dish washing, and constant human presence on paths along the rivers. In all spraint sites, except for four in the wet and five in the dry season, human activities were recorded.
As in many other otter species (L. canadensis; L. longicaudis; L. lutra; A. cinerea; A. capensis, H. maculicollis) which inhabit areas where rivers pass through cities, or scavenge around fishing boats (Kruuk, 2006; Somers, 2001), Mt. Rungwe otters are able to withstand, if not benefit from some degree of disturbance. They do not necessary need an undisturbed and clean habitat. Their tolerance to disturbance can be expected to change when the habitat become ecologically inadequate and their escape cover is no longer available (Macdonald and Mason, 1990).
Sao Hill
This was the first time that A. capensis has been investigated in Sao Hill. Density and frequency values were slightly higher (0.94-1.6 spraints/km) than recorded in Mt. Rungwe (0.86-0.88 spraints/km) where there was more human disturbance. Tourism, fishing, canoeing and timber collection did not seem to have an impact on A. capensis presence.
Sightings of H. maculicollis occur in early mornings or at night while fishing, and it was captured on our camera traps (set on a 24hr interval) on the shore at night (De Luca et al., unpublished data). Its presence in the lakes, marshes and swamps of Sao Hill, is a new distribution record and the most southern record in Tanzania before Lake Nyasa. Tanzania has many wetlands with fish that could support H. maculicollis, suggesting that species distribution may still be underestimated.
Although not statistically significant, the density estimate of H. maculicollis spraint sites in Sao Hill was higher (2.76/km) in the wet season than the dry. The lakes had greater densities of H. maculicollis spraint sites than marshes probably due to fish abundance. From interviews with fishermen and naturalists, H. maculicollis is often seen in large lakes and swamps in groups of 2 to 10 individuals in both seasons. One naturalist (S. Johnson pers. comm.) once counted 14 otters together on Lake Ngwazi.
The most suitable habitats for H. maculicollis are open water such as large lakes (Rowe-Rowe and Somers, 1998), where fish availability affects abundance and density (Procter, 1963; Kruuk & Goudswaard, 1990; Lejeune and Frank, 1990). In this study, we found that the majority of signs were on floating grass islands in lakes, and on lakeshores and swamps. The value of these islands was supported by fishermen’s observations. Each day otters are seen catching fish in the lake, often in the fishnets, and returning to islands to eat, and shelter. After fish availability, the occurrence of dense vegetation cover for resting or dens is the other limiting factor, whether otters are on floating islands, lakeshores, streams or river banks (Rowe-Rowe,1992; Perrin and Carugati, 2000b). In Sao Hill preliminary observations revealed that 72% of spraints contained only fish from Lake Inzivi and 28% contained fish and crabs from Lake Kyanga. This would indicate less availability in Lake Kyanga.
Sao Hill threats to otters
Fishing and the retaliatory trapping of otters are the main threats in Sao Hill, especially near the Ruaha Marsh. Overfishing may affect prey availability and otters are seen as competing with humans. Fishing was common in some river sections and amongst the bigger lakes but not in tea estate dams where it is forbidden. Lake Ngwazi was the most intensely fished (50-100 fishermen) whereas the smaller Mkewe swamp was less used by fishermen. Fishing techniques included traps, lines and nets set over a period of at least 24hrs. All fishermen questioned perceived otters as a threat to livelihoods. Many denied hunting otters, however, in the dry season we encountered 12 snap traps, placed in water or in the bank next to otters paths and spraint sites in Ruaha Marsh, and one had a dead H. maculicollis in it. According to fisherman, catching otters is difficult and only between 0-3 otters per year were killed. Undoubtedly this figure is underestimated.
Other proximal human activities included cattle grazing and mud collection for bricks. These threaten otters indirectly as they can alter habitat and escape cover. Little cultivation occurred right to the riverbanks, and dense, high herbaceous or shrubland vegetation often covered them. Nevertheless, cultivation was often close and the burning of riverbanks for ploughing was observed in the dry season. Our interviews revealed that the tea estate companies apply herbicides twice a year, and fertilizer once a year but no information on the amount of pesticide use was provided.
Human activities such as farming and pollution can affect A. capensis more due to the species reliance on detritivorous freshwater crabs (Perrin and Carugati 2000a,b; Rowe-Rowe, 1977a,b; Somers and Purves, 1996; Mason and Rowe-Rowe, 1992) rather than the more piscivorous H. maculicollis. Even if otters are able to tolerate disturbance they still remain vulnerable because cubs take a year to become independent (Larivière, 2001), and as with other mustelids have short reproduction spans (Kingdon, 1997; Weigl, 2005). Recovery from even a small increase in mortality or an increase in natural predation, is slow.
This study indicated that A. capensis and H. maculicollis populations in southwest Tanzania can thrive as long as the main threats are managed. Monitoring using the same sites should provide information on the status of these species through time. Persecution and hunting should be prevented, especially in Sao Hill, and in both sites the deterioration of habitat quality in escape cover should be carefully avoided.
Acknowledgements: This study was funded by the Wildlife Conservation Society. The authors would like to thank H. Jacques, C. Carugati, I. d’Inzillo Carranza, J. Reed-Smith and T. Davenport for helpful advice with survey methodology and the preparation of the manuscript. S. Markes and N.Kimambo assisted with the preparation of the maps. Field assistance was provided by S. Kimiti, A. Kajigili, W. Mwalwengele, B. Kalasa and K. Msekwa. In Sao Hill at Fox Farm we thank G. Fox, C. Congdon and S. Johnson for information on otter sightings and ecology. Finally, we are grateful to two anonymous reviewers whose comments helped improve the quality of this manuscript.
REFERENCES
Amulike, B., Stevens, S.S., Serfass, T.L. (2013). Enhancing tourist opportunities to view spotted-necked otters (Lutra maculicollis) at Rubondo Island National Park: can the a priori location of latrines simplify identifying best viewing areas. Afr. J. Ecol., 51: 609-617.
Butler, J.R.A., Du Toit, J.T (1994). Diet and conservation status of cape clawless otters in eastern Zimbabwe. S. Afr. J.Wild. Res. 24:3, 41-47.
Carss, D.N. and Parkinson, S.G. (1996). Errors associated with otter Lutra lutra faecal analysis.1: Assessing general diet from spraints. Journal of Zoology, 238 (2): 301–317
Davenport, T.R.B., De Luca, D.W., Bracebridge, C.E., Machaga, S.J., Mpunga, N.E., Kibure, O. Abeid, Y.S. (2010) Diet and feeding patterns in the kipunji (Rungwecebus kipunji) in Tanzania’s Southern Highlands: a first analysis. Primates 51: 213-221.
De Luca, D.W., Mpunga, N.E. (2004). Carnivore communities in two montane forests in southern Tanzania: inventory and ethnomammalogy in sites of differing management regimes. In: Abstracts of the 18th conference of the Society for Conservation Biology, New York.
De Luca, D.W., Mpunga, N.E. (2005). Carnivores of the Udzungwa Mountains: presence, distribution and threats. Unpublished report for the Wildl. Cons. Soc. pp. 38.
De Luca, D.W., Davenport, T.R.B., Mpunga, N.E. (2006). The conservation status of small to medium carnivores across Southern Tanzania, A discussion document for Small to Medium Carnivores workshop, Arusha 19th-21st 2006, Tanzania Carnivore Centre, Tanzania Wildlife Research Institute (TAWIRI).
De Luca, D.W., Mpunga, N.E. (2012). Carnivores of the Mt. Rungwe-Kitulo landscape, Southwest Tanzania: presence, distributions and threats. Wildl. Cons. Soc. pp 33.
De Luca, D.W., Mpunga, N.E (2013). Small carnivores of the Mt. Rungwe-Kitulo landscape, southwest Tanzania: presence, distributions and threat. S. Carn. Cons. 48, 67-82.
De Luca, D.W., N.E. Mpunga (2018). Leopard distribution and food habits in the Mt. Rungwe-Kitulo landscape, Southern Tanzania. Afr. J. Ecol. 56, 358-367.
D’Inzillo Carranza, I., Rowe-Rowe, D.T. (2013). Spotted-necked Otter, (Hydrictis maculicollis). pp114-118 in Kingdon, J.and Hoffman,M.(eds) Mammals of Africa,V. Carnivores, pangolins, equids and rhinoceroses. Bloomsbury, London, UK
Foley C., Foley L., Lobora A., De Luca D., Msuha M, Davenport T.R.B., Durant, S. (2014). A field guide to the larger mammals of Tanzania, Princeton University Press, Pp:114-116.
Foster-Turley P, Macdonald, S., Mason, C.F. (1990). Otters, an Action plan for their conservation IUCN/SSC Otter Specialist Group pp1- 126.
Jacques, H., Reed-Smith, J., Somers, M.J. (2015). Aonyx capensis. The IUCN red list of Threatened Species 2015. Downloaded on 19 September 2016.
Kingdon, J. (1997). The Kingdon field guide to African Mammals. Academic Press. Ltd. London.
Kubheka, SP, Rowe-Rowe, DT, Alletson, J.D., Perrin, M.R. (2012). Possible influence of increased riparian activity (stream modification and agricultural intensification) on abundance of South African otters. Afr. J. Ecol. 51, 288-294.
Kruuk, H., Conroy, J.W.H. (1987). Surveying otter (Lutra lutra) populations: A discussion of problems with spraints. Biol. Cons., 41, 3, 179-183.
Kruuk H., Goudswaard, P.C. (1990). Effects of changes in fish populations in Lake Victoria on the food of otters (Lutra maculicollis Schinz) and (Aonyx capensis Lichtenstein). Afr. J. Ecol. 28:322-329.
Kruuk, H. (1992). Scent marking by otters (Lutra lutra) signaling the use of resources. Behav. Ecol.,3 133-140.
Kruuk, H. (2006). Otters: ecology behaviour and conservation. Oxford University Press. Pp.265
Larivière, S. (2001). Aonyx capensis. Mammalian Species: Number 671: pp. 1 – 6
Lejeune, A., Frank, V. (1990). Distribution of Lutra maculicollis in Rwanda: ecological constraints. Otter Specialist Group Bulletin 5:8-16.
Macdonald, S., Mason, C.F. (1990). Threats. In: Otters, an action plan for their conservation. Ed. P. Foster-Turley, S. Macdonald and C. Mason IUCN/SSC Otter Specialist Group. pp 11-14.
Mason, C.F., Macdonald, S. (1986). Otters. Ecology and Conservation. Cambridge University Press.
ISBN: 9780521307161
Mason C.F., Macdonald, S. (1987). The use of spraints for surveying otter (Lutra lutra populations: an evaluation. Biol. Cons., 41, 3, 167-177.
Mason, C.F, Rowe-Rowe D.T. (1992). Organochlorine pesticide residues and PCBs in otter scats from Natal. S. A. J. Wild. Res. 22: 29-31
Nel, J.A.J., Somers, M.J. (2002). The status of otters in Africa: an assessment. Pp258-266 In Otter conservation- an example for a sustainable use of wetlands. Proceedings of the VIIth International Otters Symposium. Eds. R. Dulfer, J. Conroy, J. Nel, A.C. Gutleb. IUCN Otter Specialist Group Bulletin Vol.19A (Special Issue).
Nel, J.A.J., Somers, M.J. (2007) Distribution and habitat choice of Cape clawless otters, (Aonyx capensis), in South Africa. S. Afr. J. Wildl. 37:61-70
Perrin, M.R., Carugati, C. (2000a), Habitat use by the cape clawless otter and spotted-necked otter in KwaZulu-Natal Drakensberg, South Africa. S. Afr. J.Wildl.Res. 30(2):103-113.
Perrin, M.R., Carugati, C. (2000b) Food habits of coexisting Cape clawless otter and spotted-necked otter in the KwaZulu-Natal Drakensberg, South Africa, S. Afr. J. Wildl.Res .30(2):85-94
Perrin, M.R., Carugati, C. (2006) Abundance estimates of the Cape clawless otter (Aonyx capensis, Schinz 1821) and the spotted necked otter (Lutra maculicollis, Lichtensterin 1835) in the Kwa Zulu-Natal Dakenberg, SA. Trop. Zool. 19:9-19.
Perrin, M.R., D’Inzillo Carranza, I. (2000) Activity Patterns of Spotted-Necked Otters in the Natal Drakensberg, South Africa. S. Afr. J. Wildl.Res. 30: 1-7
Perrin, M.R., D’Inzillo Carranza, I., Linn, I.J. (2000) Use of space by the Spotted-Necked Otter in the KwaZulu-Natal Drakenberg, South Africa. S. Afr. J. Wildl. Res. 30: 15-21
Pettorelli, N, Lobora, AL, Msuha, MJ, Foley, C, Durant, SM; (2010) Carnivore biodiversity in Tanzania: Revealing the distribution patterns of secretive mammals using camera traps. Animal Conservation , 13 (2): 131-139
Procter, J. (1963). A contribution to the natural history of the Spotted-Necked Otter (Lutra maculicollis, Licht.) in Tanganyika. E. Afr. Wildl. J. 1: 93-102
Reed-Smith, J, Jacques, H., Somers, M.J. (2015). Hydrictis maculicollis. The IUCN red list of Threatened Species 2015.
[Available from
https://www.iucnredlist.org/species/12420/21936042 Downloaded on 19 September 2016. ]
Rowe-Rowe, D.T. (1977a) Food Ecology of otters in Natal, South Africa. Oikos 28:210-219.
Rowe-Rowe, D.T. (1977b). Prey capture and feeding behavior of South African otters. The lammergeyer 23:13-21.
Rowe-Rowe, D.T. (1986). African Otters-is their existence threatened? IUCN Otter Specialist Group Bulletin 1: 9-11.
Rowe-Rowe, D.T. (1992). Survey of South African otters in a fresh-water habitat, using signs. S. Afr. J. Wild. Res. 22:49-55.
Rowe-Rowe, D.T., Somers, M.J. (1998). Diet, foraging behaviour and coexistence of African otters and the water mongoose. Symp. Zool. Soc. Lond. 71, 216-227.
Somers, M.J., Purves, M.G. (1996). Trophic overlap between three syntopic semi-aquatic carnivores: Cape clawless otter, spotted-necked otter and water mongoose. Afr. J. Ecol. 34:158-166.
Somers, M.J. (2001). Habitat utilization of Cape clawless otter (Aonyx capensis). Thesis (PhD). University of Stellenbosch.
Somers, M.J., Nel J.A.J. (2013). Clawless Otter, (Aonyx capensis). pp104-108 in Kingdon, J., Hoffman, M. (eds) Mammals of Africa, V. Carnivores, pangolins, equids and rhinoceroses. Bloomsbury, London, UK.
Weigl, R. (2005). Longevity of Mammals in Captivity; from the Living Collections of the World. Kleine Senckenberg-Reihe 48: Stuttgart
Le Statut de Conservation des Loutres dans le Sud-Ouest de la Tanzanie
Deux espèces de loutre sont présentes en Tanzanie, la loutre à joues blanches (Aonyx capensis) et la loutre à cou tacheté (Hydrictis maccullicolis). Toutes deux sont considérées comme «quasi menacée» sur la liste rouge de l’IUCN, avec les principales menaces liées aux pressions exercées sur l’habitat et les ressources en nourriture de par la croissance de la population humaine. Malgré le peu d’information sur leur distribution en Tanzanie, notre travail récent montre que les deux espèces sont davantage présentes qu’on ne le pensait précédemment. Nous avons étudié la distribution et l’état de conservation de deux zones fortement peuplées dans le sud-ouest de la Tanzanie, le Mt Rungwe et la colline de Sao.
Les relevés en période pluvieuse et en saison sèche sur les sites de marquage comprenaient des rivières, des lacs, et des marais, avec des informations sur l’alimentation et la typologie des habitats. Les résultats ont mis en évidence que la présence et la distribution des espèces ne sont pas significativement affectées par la saison et qu’elles peuvent se développer aussi longtemps qu’une chasse repressive peut être évitée. La qualité de l’habitat devrait être l’objet d’un suivi afin d’éviter la détérioration du couvert végétal protecteur. La nature des menaces et la conservation des deux espèces sont analysées
Revenez au dessus
Resumen: El Estado de Conservación de las Nutrias en el Sudoeste de Tanzania
En Septiembre de 2013, fue avistado un individuo de una de las especies de nutria más elusivas y amenazadas, la Nutria de Sumatra (Lutra sumatrana), en En Tanzania hay dos especies de nutrias confirmadas, la nutria africana sin garras (Aonys capensis) y la nutria de cuello manchado (Hydrictis maculicollis). Ambas están listadas como “casi amenazadas” en la Lista Roja de la UICN, estando las principales amenazas ligadas a las presiones ejercidas en su hábitat y recursos alimentarios por una población humana creciente. A pesar de los muy escasos detalles disponibles sobre sus distribuciones en Tanzania, nuestro trabajo reciente muestra que ambas especies están más ampliamente distribuidas que lo que se creía previamente. Investigamos la distribución y estado de conservación en dos áreas altamente pobladas en el sudoeste de Tanzania, el Monte Rungwe y Sao Hill. Los relevamientos tanto en estación lluviosa como seca, buscando sitios con fecas y marcas, incluyeron ríos, lagos y pantanos, colectando datos sobre items alimentarios y rasgos del hábitat. Los resultados indicaron que la presencia de las especies y la distribución no están significativamente afectadas por la estacionalidad, y que pueden prosperar en tanto se evite la caza retaliatoria. Se debería monitorear la calidad del hábitat para evitar el deterioro de la cobertura para escape. Se discuten la naturaleza de las amenazas, y la conservación de ambas especies.
Vuelva a la tapa