IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Citation: Kanekal, N.A., Johnson, J.A., and Hussain, S.A. (2025). Distribution and Diet of Smooth-Coated Otters (Lutrogale perspicillata) in Tungabhadra Otter Conservation Reserve, Karnataka. IUCN Otter Spec. Group Bull. 42 (3): 151 - 160
Distribution and Diet of Smooth-Coated Otters (Lutrogale perspicillata) in Tungabhadra Otter Conservation Reserve, Karnataka
Niyaz Ahamed Kanekal*, Jeyaraj Antony Johnson, and Syed Ainul Hussain
Wildlife Institute of India, Chandrabani, Dehradun, India - 248001
*Corresponding Author Email: aniyaz533@gmail.com
Received 22nd April 2025, accepted 11th July
Abstract: The Smooth-coated otter (Lutrogale perspicillata), is a top aquatic predator of Indian rivers, and is listed as Vulnerable on the IUCN Red List due to habitat degradation and declining populations. This study assessed the distribution and diet of the species in the Tungabhadra Otter Conservation Reserve (TOCR), a critical 34km stretch of the Tungabhadra River. Otter distribution was analyzed using sign surveys across 500 x 500m grids, recording direct sightings and indirect signs. The spatial distribution of otters was mapped using sign density and spatial autocorrelation analysis using Moran’s I test. Otter diet was examined through spraint analysis, identifying prey species using scale and skeletal remains. A total of 132 otter signs were recorded, indicating a strongly clustered distribution (Moran’s I = 0.99203). Spraint analysis (n=92) revealed a fish-dominated diet (97.87%), with Oreochromis mossambicus (37.70%) and Cyprinus carpio (11.48%) being the most consumed species. The findings indicate that otters preferentially inhabit specific areas within TOCR and depend largely on non-native fish species. This study provides insights into otter ecology in the region and highlights the importance of habitat conservation and fisheries management for otter conservation.
Keywords: Freshwater predator, spatial ecology, non-native species, spraint analysis, mustelids.
INTRODUCTION
Freshwater ecosystems, despite constituting only 0.01% of the world’s water, are among the most biodiverse habitats on Earth, supporting at least 6% of all known species, including a wide range of aquatic and semi-aquatic organisms (Dudgeon et al., 2006). These ecosystems provide critical ecological services, such as water filtration, flood regulation, and habitat provision for numerous species, including otters. However, due to increasing anthropogenic pressure such as habitat destruction, pollution, and climate change, freshwater ecosystems are now among the most threatened ecosystems on the planet (Vörösmarty et al., 2010).
Otters, belonging to the sub-family Lutrinae of the family Mustelidae, (Ewer, 1973), are one of the most charismatic and ecologically significant groups of aquatic predators. The Mustelidae family is one of the largest families within the order Carnivora, comprising a diverse array of species adapted to various ecological niches (Mason and Macdonald, 1986). Lutrinae encompasses a total of 13 species of otters distributed around the globe except for Australia, Antarctica, and some islands (Hussain and Choudhary, 1997). Indian subcontinent has three species of otter, the Smooth-coated otter (Lutrogale perspicillata, Geoffroy 1826), the Asian small-clawed otter (Aonyx cinereus, Illiger 1815), and the Eurasian otter (Lutra lutra, Linnaeus, 1758) (Pocock, 1941; Mason and Macdonald, 1986; Hussain and Choudhury, 1997).
The smooth-coated otter (SCO) is one of the indicator species for freshwater ecosystems, found across a wide range of habitats in Asia, from the Indian subcontinent to Southeast Asia, with an isolated population in Iranian marshes (Pocock 1941). They are distributed in Indian waters from the Himalayas to Peninsular India and inhabit freshwater and brackish water habitats (Basak et al., 2021). In India, despite their ecological importance, SCO is particularly vulnerable due to the rapid transformation of freshwater habitats for agriculture, urbanization, and industrial development. As a result, otter populations are now largely confined to protected areas, such as the Tungabhadra Otter Conservation Reserve (TOCR) in Karnataka. However, despite the establishment of TOCR, there is a lack of comprehensive studies on the ecology of SCO in this region. This study seeks to address two fundamental ecological questions concerning SCO in TOCR: their distribution pattern and diet composition.
By answering these questions, this study will provide critical insights into the ecology of SCO in semi-arid river ecosystems, contributing to the development of effective conservation strategies for this vulnerable species. Furthermore, the findings will have broader implications for the management of freshwater ecosystems in India and other regions facing similar ecological challenges.
MATERIALS AND METHODS
Study Site
The Tungabhadra Otter Conservation Reserve (TOCR), a 34 km stretch of Tungabhadra river in Karnataka, was notified as a conservation reserve in 2015 and it extends from the village of Mudlapur to Kampli in Ballari district (15°15'44.01" N, 76°20'17.67" E to 15°26'27.90 "N, 76°36'58.84 "E, Fig. 1), primarily for the conservation of Smooth-coated otters. This protected stretch lies just downstream of Tungabhadra reservoir, leading to restricted water flow, with little to no flow throughout the year, resulting in largely stagnant or isolated pools along the river stretch. The area lies within the semi-arid part of the Deccan plateau. According to Champion and Seth classification the vegetation of this conservation reserve belongs to Dry Deciduous Scrub (5DS1) and Southern thorn forests (6A/DS1). Apart from Smooth coated otters, other fauna of TOCR include Marsh Crocodile (Crocodylus palustris), Leith’s softshell turtle (Nilssonia leithii), Indian narrow headed softshell turtle (Chitra indica), deccan mahseer (Tor khudree), and tunga garra (Garra bicornuta) (Ministry of Environment, Forest and Climate Change, 2019).
Sampling Strategy
Distribution Pattern
The entire stretch of TOCR was systematically surveyed from December 2023 to March 2024 using 500 x 500 m grids. Surveys were conducted intensively by foot and boat during the otters peak activity periods to record direct sightings and indirect signs of SCO. The choice of a 500 x 500 m grid size was based on an initial reconnaissance survey conducted from October 26 to October 29 – 2023, which used coarser 1 x 1 km grid size. This preliminary survey recorded an average spraint density of only 3 spraints per 1 x 1 km grid size, making it challenging to establish a gradient of grids. To improve resolution, the approach was focused on assessing overall sign density rather than just spraint density. The Locus mobile application was used to track movement during sign surveys and mark waypoints for direct sightings and indirect signs.
Diet Composition
Preparation of the Standard Reference Collection of Fish for Spraint Analysis
The preparation of the standard references and fish collection was carried out with the assistance of local fisher folk. Over a month, we visited seven fish landing sites around the Tungabhadra reservoir, collecting 2-4 individuals of each species with varying lengths. The fish species were selected based on the Ichthyofaunal list of the Tungabhadra River, compiled from the existing literature (Nagabhusan, 2022).
Each fish species exhibits unique characteristics such as scale shape, scale pattern, scale line in anterior and posterior sides which can be used later for identification using spraints of SCO (Webb, 1976; Bräger and Mortiz, 2016). While the size and texture of scales vary across different regions of a fish’s body, the shape remains consistent. To account for this variation, scales were collected from three specific regions: (i) the base of head, (ii) above the lateral line, and (iii) near the tail. The scales were soaked in the distilled water and gently scraped with a small brush to remove the mucous layer. They were then stained using Alizarin Red S dye, mounted with DPX, sealed with a coverslip, and labelled for future reference in spraint analysis. For species lacking scales, such as catfish and eels, specimens were boiled for 15-20 minutes to separate muscle from bones. The dorsal spine and razor-ray fin bones were then isolated, dried, and preserved for identification.
Collection of Spraints
During the sign survey about 45 communal spraint sites were identified and spraints were collected from these sites. Fresh and recent spraints, about 1- 2 days old were collected and very old spraints were not collected. The spraint’s age was identified based on characteristics such as sliminess, color, and smell. Fresh spraints were dark grey color, fully intact, stiff, and had a pungent smell. In contrast, very old spraints turned completely white and scattered. A total of 92 spraint samples were collected from the field, sundried, and the dry weight of each sample taken using a weighing balance (d=0.01g, e=0.01g).
Sorting and Identification of Prey Items
Each spraint sample was carefully placed on a gridded petri dish for detailed analysis. Non-digested prey remains, including scales, bones, and exoskeleton fragments, were identified and classified as either fish or crab. Given the time-intensive nature of examining all scales and remains, a sub-sampling approach was implemented. Specifically, ten scales were randomly selected from different sections of the petri dish. If all ten scales belonged to the same species, the spraint was recorded as containing only that species. However, if a scale from a different species was identified, an additional ten scales were randomly selected, and the process was repeated until all species present in the spraint were accurately identified (Basak et al., 2021). Following identification, the scales were stained and examined under a compound microscope at 4X magnification for further analysis.
Analysis
Distribution Pattern
After completing the sign survey, sign density per grid was calculated, as follows:
| Sign density (signs/m2) = Number of signs per grids (N) / Total distance covered per grid (m) |
The total distance covered in each grid, representing survey effort was quantified using the Intersect tool in ArcGIS, which calculated the sum of all tracks (in meters) within each grid. Based on the sign density values, the grids were divided into tertiles (three equal proportions of data) to classify them as high-sign density grids [HD] (>66%), moderate-density grids [MD] (33-66%), and low-density grids [LD] (<33%). These tertiles were then used to map the distribution of SCO in the TOCR. To analyze the data, normality was assessed using the Shapiro-Wilk test, and, based on the results, the Kruskal-Walis test was applied to compare otter sign densities across the different density sites. Additionally, Moran’s I test was used to evaluate the spatial distribution of otter signs and determine whether they were clustered, dispersed, or randomly distributed across the study area. This approach provided a robust framework for understanding the spatial patterns within the reserve.
Diet Composition: Estimation of Proportions of Prey Items Consumed
Two methods (frequency of occurrence and score bulk estimate method) were used to express the data obtained from spraint analysis. The most common is determining the Frequency of Occurrence. However, this can lead to overrepresentation of minor items and underestimation of major ones. To address this issue, a visual scoring method called Score Bulk Estimate can be used to assess the importance of a particular item in a spraint.
Frequency of Occurrence: The prey categories present in each spraint were identified and the relative frequency of each prey item was calculated i.e. the number of occurrences of a prey category was expressed as the percentage of samples having that category. This method of scat analysis is implemented in many of the studies (Erlinge, 1968 for the Eurasian otter; Melquist and Horncocker, 1983 for the North American river otter; Hussain and Choudhary, 1998 for the smooth-coated otter; Pardini, 1998 for Neotropical river otter; Perrin and Carugati 2000 for spot-necked otter and Cape clawless otter).
Score-bulk estimate: The proportion of each prey category was visually estimated and given a score from 1 to 10 on the coverage in the Petri dish. Then the score of each prey category was then multiplied with the dry weight of the spraint and the resulting figures were summed up for each prey category and expressed as a percentage (Wise et al., 1981; Hussain and Choudhary, 1997).
RESULTS
Distribution Pattern
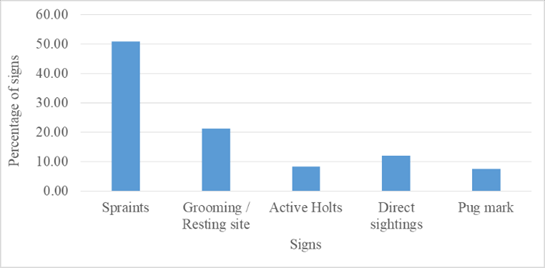
An extensive survey spanning 102 km was carried out on foot and boat along the 34 km stretch of TOCR to identify direct sightings and indirect signs of SCO. Over the course of the study, 132 signs of SCO were documented within the 34 km area. A detailed breakdown of different types of signs observed is provided in Figure 2.
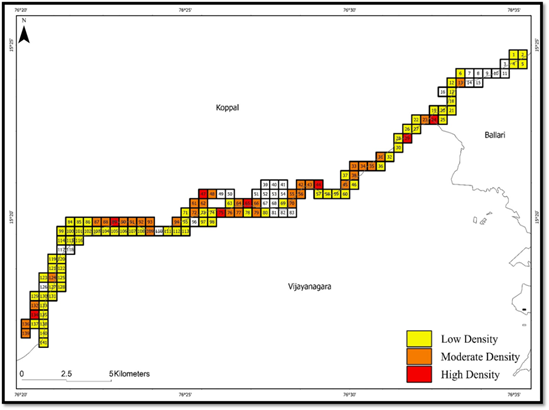
The distribution pattern displayed a clustered arrangement on the map, and the Moran’s I test revealed a highly significant positive spatial autocorrelation with a value of 0.99203, nearing +1. This indicates that otter signs are strongly clustered rather than randomly dispersed across the TOCR. Consequently, the findings highlight a significant clustering of otter signs, implying that SCO exhibit a non-random, aggregated distribution pattern in this region (Figure 3).
Diet Composition
The analysis of otter spraints using the ‘frequency of occurrence’ method revealed that fish constituted the dominant component of the otter diet, accounting for 97.87%, followed by crabs at 2.13%. Further examination of the fish species identified in the spraints, using both the ‘frequency of occurrence’ and ‘score-bulk estimate’ methods, showed a total of 14 fish species, with one remaining unidentified as in Table 1. Among these, Oreochromis mossambicus made the highest contribution (FOC=37.70%, SBE=39.77%), followed by Cyprinus carpio (FOC=11.48%, SBE=12.32%). These findings indicate that the diet of SCO in the TOCR is not uniformly distributed across prey species. Instead, it is heavily dominated by specific species, particularly Oreochromis mossambicus and Cyprinus carpio.
DISCUSSION
Distribution Pattern
Sign surveys, which measure spatial patterns of animals based on the detection or non-detection of animal signs (Heinemeyer et al., 2008), are non-invasive and cost-effective methods for studying animal distribution (Humphrey and Zinn, 1982). In this study, sign surveys revealed that the SCO in the TOCR exhibit a clumped distribution pattern. High-density areas identified within the reserve serve as core regions of otter activity, suggesting that otters preferentially select specific areas within their habitat. This clumped distribution is likely driven by the presence of optimal habitat features, such as abundant food resources, adequate escape cover, suitable water quality, minimum human disturbance, and favorable shelter sites – all of which are critical for otter survival and reproduction.
The clumped distribution pattern observed in this study aligns with findings from previous research on otter populations. For instance, Jenkins and Burrows (1980) reported that Eurasian otter (Lutra lutra) spraint densities were non-random and clumped, a pattern attributed to habitat preferences and resource availability. Similarly, Mason and Macdonald (1986) documented significant clustering of otter spraints in British river systems, particularly around specific habitat features such as large rocks and tree roots. Kruuk (2006) further emphasized that otter populations tend to aggregate in areas with high prey availability and low human disturbance, reinforcing the clumped distribution pattern observed in this study. Additionally, Romanowski et al. (2013) found that Eurasian otters preferentially selected river stretches with higher fish densities and lower pollution levels, resulting in non-random, clumped distributions. These parallels across studies highlight the consistency of otter habitat preferences and the influence of ecological factors on their spatial distribution.
The identification of high-density otter activity areas in the TOCR has important implications for conservation and management. Prioritizing the protection of these core regions is essential for ensuring the survival and reproductive success of the SCO population. Conservation strategies should focus on maintaining optimal habitat conditions, such as preserving water quality, ensuring adequate prey availability, and minimizing human disturbance. By safeguarding these high-density areas, the forest department can enhance the long-term viability of the otter population within the reserve.
In conclusion, the clumped distribution pattern of SCO in TOCR underscores the importance of targeted conservation efforts. Protecting high-density otter activity areas will not only support the ecological requirements of the species but also contribute to the overall health and biodiversity of the reserve’s aquatic ecosystems.
Diet Composition
This study highlights the strong reliance of SCO on fish, confirming their predominantly piscivorous diet, which constitutes approximately 98% of their food intake, with crabs making up the remaining 2%. These findings align with previous studies conducted in other regions, which also reported a fish-dominated diet for SCO (Hussain, 2013; Anoop and Hussain, 2005; Nawab and Hussain, 2012; Basak et al., 2021). Notably, the absence of birds, amphibians, or mammals in the otter’s diet during the study period suggests that fish and crabs alone are sufficient to meet their energy requirements. This observation is consistent with earlier research, which emphasised the occasional consumption of secondary prey categories such as crabs (Anoop and Hussain 2005; Basak et al., 2021; Baskaran et al., 2021).
In the TOCR, the diet of otters is composed of approximately 69.53% non-native fish species, including Oreochromis mossambicus, Cyprinus carpio, Labeo rohita, and Cirrhinus cirrhosis. This reflects the high local availability of non-native fish compared to native species. The introduction of non-native and invasive fish species by the National Fisheries Development Board (NFDB) has significantly altered the natural Ichthyofaunal assemblages in these ecosystems. While these introductions aim to achieve specific objectives, they often overlook the long-term ecological impacts on native fish communities (Milardi et al., 2019). The dietary diversity of carnivores, including SCO, is known to fluctuate inversely with the prey availability (Tinker et al., 2008). This is evident in the current study, where over 60% of the otter’s diet consists of just three non-native fish species, underscoring the influence of prey availability on dietary composition.
The study employed two methods to estimate diet composition: the ‘frequency of occurrence’ method and the ‘score-bulk estimate’ method. Each method has its strengths and limitations. The frequency of occurrence method tends to overestimate the importance of prey items present in small quantities while underestimating those present in larger amounts, potentially leading to a skewed interpretation of dietary significance. This issue has been widely criticized in previous research (Jacobsen and Hansen 1996). Conversely, the score-bulk estimate method, though more subjective due to its reliance on visual estimation and scoring, provides a more accurate representation of prey contribution to the diet. Despite the subjectivity, the study found a relatively consistent percentage (+/-5%) of prey categories across both methods. Based on these findings, it is recommended that the score-bulk estimate method offers a more reliable estimate of prey composition compared to other methods (Jacobsen and Hansen 1996).
Overall, this study underscores the adaptability of SCO to changes in prey availability, particularly the shift towards non-native fish species. However, the long term ecological consequences of such dietary shifts, driven by human-induced alterations to aquatic ecosystems remain poorly understood and could potentially disrupt native food webs.
Acknowledgements: We extend our gratitude to the Karnataka Forest Department for timely provision of research permits and facilitation. We extend our gratitude to the Director and Dean of the WII for their assistance and cooperation in the smooth conduct of the study.
REFERENCES
Anoop, K. R., and Hussain, S. A. (2005). Food and feeding habits of smooth-coated otters (Lutra perspicillata) and their significance to the fish population of Kerala, India. J. Zool., 266(1), 15–23. https://doi.org/10.1017/s0952836905006540
Basak, S., Pandav, B., Johnson, J. A., and Hussain, S. A. (2021). Resource utilisation by smooth-coated otter in the rivers of Himalayan foothills in Uttarakhand, India. Glob. Ecol. Conserv., 32, e01896. https://doi.org/10.1016/j.gecco.2021.e01896
Baskaran, N., Sundarraj, R. S., and Sanil, R. (2022). Population, distribution and diet composition of smooth-coated otter Lutrogale perspicillata in Hosur and Dharmapuri Forest Divisions, India. J. Threat. Taxa, 14(1), 20469–20477. https://doi.org/10.11609/jott.7477.14.1.20469-20477
Bräger, Z., Moritz, T., Tsikliras, A. C., Gonzalvo, J., Radulović, M., and Staszny, Á. (2016). Scale morphometry allows discrimination of European sardine Sardina pilchardus and round sardinella Sardinella aurita and among their local populations. J. Fish Biol., 88(3), 1273–1281. https://doi.org/10.1111/jfb.12907
Champion, H. G., and Seth, S. K. (1968). A revised survey of the forest types of India. Manager of Publications.
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur‐Richard, A., Soto, D., Stiassny, M. L. J., and Sullivan, C. A. (2006). Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev., 81(2), 163–182. https://doi.org/10.1017/s1464793105006950
Erlinge, S. (1968). Food studies on captive otters Lutra lutra L. Oikos, 19(2), 259. https://doi.org/10.2307/3565013
Ewer, R. F. (1973). The carnivores. Cornell University Press. ISBN13: 9780801407451
Heinemeyer, K. S., Ulizio, T. J., Harrison, R. L., Long, R. A., and MacKay, P. (2008). Natural sign: Tracks and scats. Pp. 45–74 in Long, RA, MacKay, P, Ray, J., and Zielinski, W. (Eds). Noninvasive Survey Methods for Carnivores. Island Press. ISBN-13: 978-1597261203
Humphrey, S. R., and Zinn, T. L. (1982). Seasonal habitat use by river otters and Everglades mink in Florida. J. Wildl. Manag., 46(2), 375. https://doi.org/10.2307/3808649
Hussain, S. A. (2013). Activity pattern, behavioural activity and interspecific interaction of smooth-coated otter (Lutrogale perspicillata) in National Chambal Sanctuary, India. IUCN Otter Spec. Group Bull., 30(1), 5–17. https://www.iucnosgbull.org/Volume30/Hussain_2013.html
Hussain, S. A., and Choudhury, B. C. (1997). Distribution and status of the smooth-coated otter Lutra perspicillata in National Chambal Sanctuary, India. Biol. Conserv., 80(2), 199–206. https://doi.org/10.1016/s0006-3207(96)00033-x
Hussain, S. A., and Choudhury, B. C. (1998). Feeding ecology of the smooth-coated otter Lutra perspicillata in the National Chambal Sanctuary, India. In Behaviour and ecology of riparian mammals (pp. 229–250). https://doi.org/10.1017/cbo9780511721830.015
IUCN. (2015). The IUCN Red List of Threatened Species. International Union for Conservation of Nature. https://www.iucnredlist.org
Jacobsen, L., and Hansen, H.‐M. (1996). Analysis of otter (Lutra lutra) spraints: Methods to estimate prey proportions and size of prey fish. J. Zool., 238(1), 167–180. https://doi.org/10.1111/j.1469-7998.1996.tb05387.x
Jenkins, D., and Burrows, G. O. (1980). Ecology of otters in Northern Scotland. J. Anim. Ecol., 49(3), 755. https://doi.org/10.2307/4225
Kruuk, H. (2006). Otter ecology and its background. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780198565871.003.0001
Macdonald, S. M. (1986). Otters: Ecology and conservation. Cambridge University Press. ISBN : 9780521101349
Mason, CF and Macdonald, SM (1986). Otters: Ecology and Conservation. Cambridge University Press. ISBN-13 : 978-0521307161
Melquist, W. E., and Hornocker, M. G. (1983). Ecology of river otters in west central Idaho. Wildl. Monogr., 83(1), 3–60.
Milardi, M., Gavioli, A., Soininen, J., and Castaldelli, G. (2019). Exotic species invasions undermine regional functional diversity of freshwater fish. Sci. Rep., 9(1). https://doi.org/10.1038/s41598-019-54210-1
Ministry of Environment, Forest and Climate Change. (2019). Wildlife conservation plan for Tungabhadra Otter Conservation Reserve, Karnataka. Government of India.
Nagabhushan, C. M. (2022). Conservation status of freshwater fishes reported from Tungabhadra Reservoir, Karnataka, India. J. Threat. Taxa, 14(8), 21704–21709. https://doi.org/10.11609/jott.7593.14.8.21704-21709
Nawab, A., and Hussain, S. A. (2012). Factors affecting the occurrence of smooth-coated otter in aquatic systems of the Upper Gangetic Plains, India. Aquat. Conserv., 22(5), 616–625. https://doi.org/10.1002/aqc.2253
Pardini, R. (1998). Feeding ecology of the neotropical river otter Lontra longicaudis in an Atlantic Forest stream, southeastern Brazil. J. Zool., 245(4), 385–391. https://doi.org/10.1017/s0952836998008024
Perrin, M. R., and Carugati, C. (2000). Habitat use by the Cape clawless otter and the spotted-necked otter in the KwaZulu-Natal Drakensberg, South Africa. S. Afr. J. Wildl. Res., 30(3), 103–113. https://hdl.handle.net/10520/EJC117103
Pocock, R. I. (1941). The fauna of British India, including Ceylon and Burma: Mammalia (Vol. 2).
Romanowski, J., Brzeziński, M., and Żmihorski, M. (2012). Habitat correlates of the Eurasian otter Lutra lutra recolonizing Central Poland. Acta Theriol., 58(2), 149–155. https://doi.org/10.1007/s13364-012-0107-8
Tinker, M. T., Bentall, G., and Estes, J. A. (2008). Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc. Natl. Acad. Sci. USA, 105(2), 560–565. https://doi.org/10.1073/pnas.0709263105
Vörösmarty, C. J., McIntyre, P. B., Gessner, M. O., Dudgeon, D., Prusevich, A., Green, P., Glidden, S., Bunn, S. E., Sullivan, C. A., Liermann, C. R., and Davies, P. M. (2010). Global threats to human water security and river biodiversity. Nature, 468(7321), 334. https://doi.org/10.1038/nature09549
Webb, J. B. (1976). Otter spraint analysis. Mamm. Rev., 6(1), 13–23.
Wise, M. H., Linn, I. J., and Kennedy, C. R. (1981). A comparison of the feeding biology of mink (Mustela vison) and otter (Lutra lutra). J. Zool., 195(2), 181–213. https://doi.org/10.1111/j.1469-7998.1981.tb03458.x
Résumé: Répartition et Alimentation des Loutres à Pelage Lisse (Lutrogale perspicillata) dans la Réserve Naturelle des Loutres du Tungabhadra, au Karnataka
La loutre à pelage lisse (Lutrogale perspicillata), prédateur aquatique majeur des rivières indiennes, est classée « vulnérable » sur la Liste rouge de l’UICN en raison de la dégradation de son habitat et du déclin de ses populations. Cette étude a évalué la répartition et le régime alimentaire de l’espèce dans la Réserve Naturelle des Loutres du Tungabhadra (RNLT), un tronçon critique de 34 km de la rivière Tungabhadra. La répartition des loutres a été analysée à l’aide de relevés d’indices de présence sur un maillage de 500 x 500 m, en y enregistrant les observations directes et indirectes. La répartition spatiale des loutres a été cartographiée à l’aide de la densité des indices de présence et l’analyse d’autocorrélation spatiale selon le test I de Moran. Le régime alimentaire des loutres a été étudié par analyse des épreintes, par identification des espèces de proies à l’aide des écailles et des restes squelettiques. Au total, 132 indices de présence de loutres ont été enregistrés, indiquant une répartition fortement groupée (I de Moran = 0,99203). L’analyse des épreintes (n = 92) a révélé un régime alimentaire dominé par les poissons (97,87 %) : Oreochromis mossambicus (37,70 %) et Cyprinus carpio (11,48 %) étant les espèces les plus consommées. Ces résultats indiquent que les loutres occupent préférentiellement des zones spécifiques de la région de la RNLT et dépendent fortement d'espèces de poissons non indigènes. Cette étude apporte un éclairage sur l’écologie des loutres dans la région et souligne l'importance de la conservation de l’habitat et de la gestion des pêches pour la conservation des loutres.
Revenez au dessus
Resumen: Distribución y Dieta de la Nutria Lisa (Lutrogale perspicillata) en la Reserva de Conservación de Nutrias Tungabhadra, Karnataka
La nutria Lisa (Lutrogalle perspicillata) es un predador acuático tope de los ríos de la India, y está listada como Vulnerable en la Lista Roja de UICN debido a la degradación del hábitat y poblaciones declinantes. Este estudo evaluó la disribución y dieta de la especie en la Reserva de Conservación de Nutrias Tungabhadra (TOCR en inglés), un tramo crítico de 34 km del Río Tungabhadra. Se analizó la distribución de nutrias utilizando relevamientos de signos en grillas de 500x500 m, registrando los avistajes directos, y los signos indirectos. Se mapeó la distribución espacial de las nutrias utilizando la densidad de signos y el análisis de autocorrelación espacial, con el test I de Moran. La dieta de la nutria fue examinada a través del análisis de fecas, identificando las especies presa utilizando restos de escamas y esqueletales. Se registró un total de 132 signos de nutria, indicando una distribución fuertemente agrupada (I de Moran = 0.99203). Los análisis de fecas (n=92) revelaron una dieta dominada por peces (97.87%), siendo Oreochromis mossambicus (37.70%) y Cyprinus carpio (11.48%) las especies más consumidas. Los hallazgos indican que las nutrias preferentemente habitan áreas específicas dentro de la TOCR y dependen fuertemente de especies no-nativas de peces. Este estudio proporciona perspectivas sobre la ecología de esta nutria en la región, y destaca la importancia de la conservación del hábitat y del manejo de las pesquerías, para la conservación de las nutrias.
Vuelva a la tapa